TearSolutions has developed LacripepTM, a proprietary first-in-class peptide therapy

Lacripep, which is derived from the Lacritin protein, is a first-in-class topical therapy that preserves all of Lacritin’s bioactivity.

people have signs and/or
symptoms
of Dry Eye Disease in the United States1
people are diagnosed
with
Dry Eye Disease in the United States1
people have signs and/or
symptoms
of Dry Eye Disease worldwide1
people are diagnosed
with
Dry Eye Disease worldwide1
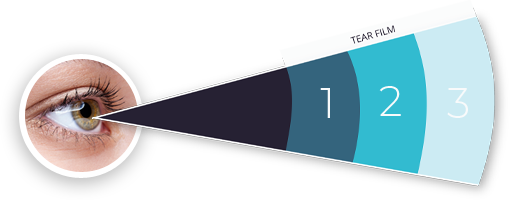
Produced by corneal epithelial cells and goblet cells on the conjunctiva.
Provides nutrients, enzymes, growth factors, etc. to help nourish the cells of the ocular surface.
Produced by the lacrimal glands.
Stabilizes the tear film by complexing with tear proteins to prevent tear film collapse.
Produced by meibomian glands in the eyelids.


Goal: Test whether Lacripep restores health to eyes of patients with ocular surface disease associated with primary Sjögren’s Syndrome (NCT03226444).
Trial Sites: ~35 private and academic clinics in 17 different states.
Status: Enrollment complete. Statistically significant improvement in both sign and symptom at two weeks.
Goal: Optimize Lacripep’s efficacy in patients with moderate/severe general dry eye.
Trial Sites: ~10 – 12 private and academic clinics expected.
Status: Clinical trial protocol design/development and fundraising.
If you are interested in participating in a clinical trial for Lacripep in Denver, CO, or St. Louis, MO, contact TearSolutions today!
TearSolutions, Inc.
315 Old Ivy Way, Suite 301
Charlottesville, Virginia 22903
TearSolutions CFO Colin Rolph:
crolph@tearsolutions.com






Ms. Carpenter is a senior management leader and regulatory advisor with more than 25 years of experience gaining global approvals for drugs and biologics for companies ranging from start-up to Fortune 500. She has extensive experience in ophthalmology, including multiple programs for dry eye disease (Santen, Otuska) in addition to a broad number of indications to treat anterior and posterior eye segment diseases. Ms. Carpenter has held numerous Senior/Vice President roles in Regulatory Affairs, Quality Affairs and Project Management and has worked at True North and Afferent Pharmaceuticals, Sanofi, Santen, and Merck. She also teaches Regulatory Affairs certification courses for the UC Santa Cruz Bioscience Program and has managed a successful consulting firm.
Robert brings more than two decades of domestic and global leadership experience in the Ophthalmology space driving successful drug development in public and private sectors through efficient organizational structure and capital investments. His widespread experience in Ophthalmology stems from diverse leadership roles across commercial, business development, medical affairs, and venture-backed startups. Currently, Robert serves as the Chief Executive Officer at AsclepiX Therapeutics focused on advancing an integrin regulating peptide for posterior segment diseases and recently led a successful Series A-3 financing. He joined Asclepix in 2021, after serving as the Chief Executive Officer for TearClear where he accelerated the business and commercial strategies including a successful Series B financing and positive FDA engagements. Prior to that, he served as the Global Head of Ophthalmology at Shire, then Takeda, responsible for one of the top ten largest biopharma M&A deals in 2019, the divestiture of Xiidra® (lifitegrast ophthalmic solution) 5%, to Novartis in June 2019 for up to $5.3B.


Mr. Asrani joined TearSolutions as President and CEO in 2020 after spending more than 9 years with Medtronic PLC, a medical device company where he held positions in marketing, investor relations, business development and strategy. Prior to joining Medtronic, Mr. Asrani lead sales and marketing for Devicix, a medical device design firm, and spent more than a decade performing academic medical research. Early in his career, Mr. Asrani worked in the UVA laboratory of Dr. Gordon Laurie, TearSolutions co-founder and current Chief Scientific Officer, and was an initial investor in TearSolutions.

Mark B. Logan is the Co-founder and Director of the Company. Mr. Logan and Dr. Gordon Laurie co-founded the Company in 2013, and he served as the President and CEO of the Company from 2013 to 2016. Prior to his work with the Company, Mr. Logan was the President, CEO and Chairman of VISX, Inc. (now a division of Johnson & Johnson). VISX invented, developed, obtained FDA approval and commercialized the procedure known as LASIK. Prior to joining VISX, he was Senior VP, COO and served on the Board of Directors of Bausch & Lomb.





Dr. John Sheppard, MD is the Mid-Atlantic Medical Director of Eye Care Partners, former Board of Directors member of CVP Physicians, the senior and founding partner of Virginia Eye Consultants, and the COO of the translational pharmaceutical development company EyeRx Research, Inc. At Eastern Virginia Medical School, Dr Sheppard serves as Professor of Ophthalmology, Microbiology, and Molecular Biology; the research director for the Ophthalmology residency program; and the clinical director of the Thomas R. Lee Center for Ocular Pharmacology and has been a recipient of the Honor and Senior Honor Awards from the American Academy of Ophthalmology. Dr Sheppard received his bachelors, master’s and Doctor of Medicine degree in the 7 year Brown University Program in Medicine on a full US Navy Armed Forces Health Professions Scholarship. He completed a pediatrics internship at the University of Virginia and a residency at the Pittsburgh Eye & Ear Institute and a cornea, uveitis, immunology and international ophthalmology fellowship at the UCSF Proctor Research Foundation.

Dr. Nancy McNamara, OD, PhD, MS is currently the Associate Dean for Academic Affairs at the University of California Berkeley (UCB), Herbert Wertheim School of Optometry and Vision Science. She received her Doctor of Optometry degree from the Michigan College of Optometry and completed a Cornea & Contact lens residency at UC Berkeley. Following
residency, Dr. McNamara earned her Ph.D. in Vision Science at UCB and embarked on a research career at the University of California, San Francisco (UCSF) where she studied the cellular and molecular mechanisms of ocular inflammation, infection, and dry eye disease. She returned to UCB to establish and co-direct the University Eye Center, Dry Eye Clinic. Dr. McNamara continues an active translational research program that includes both basic science studies to define the mechanisms underlying dry eye disease development at UCSF and clinical studies to test innovative new therapies for clinical care at UCB. She established and currently directs UCB’s Sjogren’s Clinic, a multidisciplinary care center for Sjogren’s patients, and recently joined UCSF and several top research institutions in the United States to form the Sjogren’s Team for Accelerating Medicines partnerships.
